Business Development
Our core Business Development group is strictly for venture Capital integration with new medical device start-up solutions:
Our Services are specific to:
- Technology evaluation for VC or Angel groups of new MD&D technology
- Business Plan evaluation for new MD&D start-ups.
- License/royalty/merger deals to combine multiple new MD&D start-ups to provide a stronger IP or Value Proposition
CMEDDS does not provide resources for the pharmacology sector in Business Development at this time UNLESS a company has completed Phase I, II, III and requires advanced Series C-E funding for phase IV.
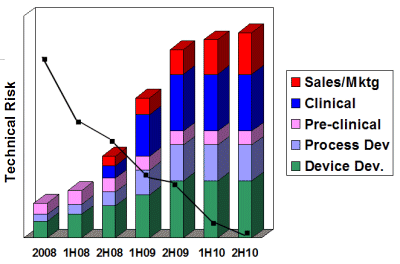
Our Risk/Analysis models are designed specifically for MD&D technology. Risk/Analysis requires validation from our consultants in Sales, Marketing, R&D, Clinical/Regulatory and Professional Education. A minimum of six weeks is needed from development to validation.